(330) 876-1228
8507 Main StreetKinsman, OH 44428
(330) 876-1229
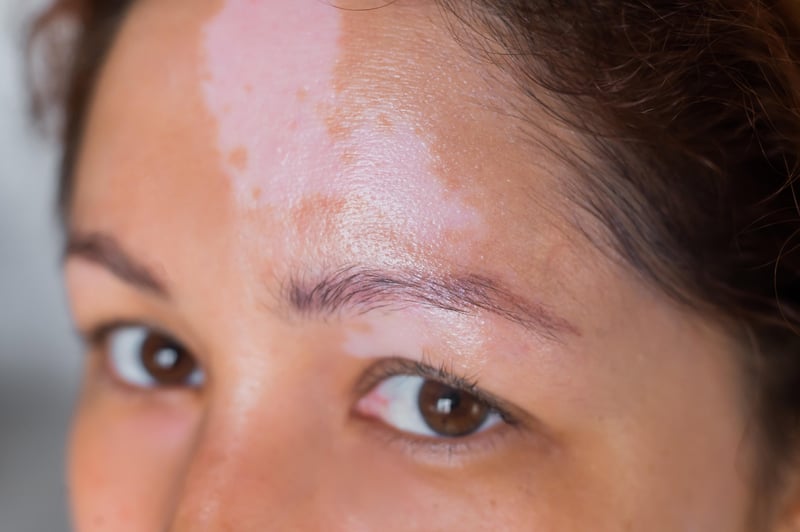
For the millions of people who live with vitiligo, a disease that robs the skin of its natural color, a newly approved cream called ruxolitinib (Opzelura) is quickly becoming a game changer.
The U.S. Food and Drug Administration approved ruxolitinib for vitiligo in people aged 12 and older in July. The drug, part of a class known as Janus kinase (JAK) inhibitors, targets JAK, a molecule involved in the development and progression of vitiligo.
About half of folks with vitiligo who used the cream got 75% or more pigment back on the face and 50% or more pigment back on their whole body after a year of use, the study found. More than one-third of adults and more than 50% of adolescents in the study said their vitiligo was no longer noticeable or a lot less noticeable after a year.
"This is a major milestone as ruxolitinib cream is the first treatment that's FDA approved to re-pigment patients with vitiligo,"said study author Dr. David Rosmarin, a dermatologist and vice chair for education and research in the department of dermatology at Tufts Medical Center in Boston.
An autoimmune disease that occurs when the body misfires against its own skin cells, vitiligo can be mild or severe and found anywhere on the body, although it typically affects the face and hands. Vitiligo affects people of all skin types but is often more noticeable in people with darker skin.
Two studies included in the new analysis involved more than 670 people with vitiligo from 70 centers, primarily in North America and Europe. There were no differences in responses based on race, ethnicity, duration of disease, and/or the amount of vitiligo, among other factors.
"Even patients with vitiligo for over 30 years can still improve with this treatment,"Rosmarin said.
Before the new cream's approval, dermatologists used topical steroids, topical calcineurin inhibitors, and phototherapy to treat vitiligo, he noted.
"Topical corticosteroids and topical calcineurin inhibitors help some patients, but certainly not everyone and [it] can also have side effects,"Rosmarin said. "Corticosteroids can lighten and thin the skin and we have to limit the use on sensitive body sites like face, genitals, and armpits, and calcineurin inhibitors can sting or burn where applied for some patients."
Phototherapy involves weekly appointments that are not always geographically convenient, he added.
Topical ruxolitinib caused only mild side effects, including redness and irritation at the application site and mild acne in this study. The medication does carry a black box warning from the FDA due to a small increased risk of serious infections, major heart issues, clotting, cancer and even death. This warning is based on studies of oral ruxolitinib, which results in much higher blood levels than the cream.
The study was funded by Incyte Corp., which produces ruxolitinib. It was published Oct. 20 in the New England Journal of Medicine.
Vitiligo experts welcome the new medication and are optimistic about the vitiligo treatment pipeline.
Vitiligo has traditionally been understudied despite its dramatic social and psychological impact, said Dr. Victor Huang. He is director of the Vitiligo Clinic at the University of California, Davis.
"The development of this new medication is exciting to the community for the treatment it offers, the validation of the underlying science of vitiligo it represents, and for the novel treatments to come it promises,"Huang said.
"This disease can take a dramatic toll on self-esteem," he explained. "Half of all patients with vitiligo are diagnosed before the age of 20, and in the pediatric population, teasing, bullying, self-consciousness and embarrassment have been issues for patients."
There's also more to it, Huang said.
"My patients report the burden of vitiligo manifesting in the time it takes to apply cover-up in the morning, difficulty with developing intimate relationships, the anxiety of always standing out, dealing with strangers who are afraid vitiligo is contagious, and time away from work to pursue treatments which can involve three-times-a-week visits for phototherapy,"according to Huang.
Dr. Iltefat Hamzavi, a dermatologist at Henry Ford Hospital in Detroit, agreed that this new cream is a big deal for people with vitiligo.
"Vitiligo is one of the most significant psychosocial diseases with an impact on quality of life similar to chronic asthma, and rates of depression are much higher than in the general population,"Hamzavi said. "We never know how well a medication can work in a large group and also how safe it is, [and] we have that information now and that is a tremendous hurdle to overcome for a disease that had no approved treatments."
Dr. Liv Eidsmo is a professor of translational skin and immunology at the University of Copenhagen, in Denmark. She wrote an editorial accompanying the new study. Topical treatment targets the affected sites directly and lowers the risk of systemic effects, she said.
"Patients with vitiligo finally have the hope of efficient treatments, with several new immuno-modulating drugs in different phases of clinical trials,"Eidsmo added.
More information
Learn more about vitiligo and its treatments at the American Academy of Dermatology.
SOURCES: David Rosmarin, MD, dermatologist, vice chair, education and research, dermatology, Tufts Medical Center, Boston; Victor Huang, MD, associate professor and director, Vitiligo Clinic, University of California, Davis; Iltefat Hamzavi, MD, dermatologist, Henry Ford Hospital, Detroit; New England Journal of Medicine, Oct. 20, 2022
