(330) 876-1228
8507 Main StreetKinsman, OH 44428
(330) 876-1229
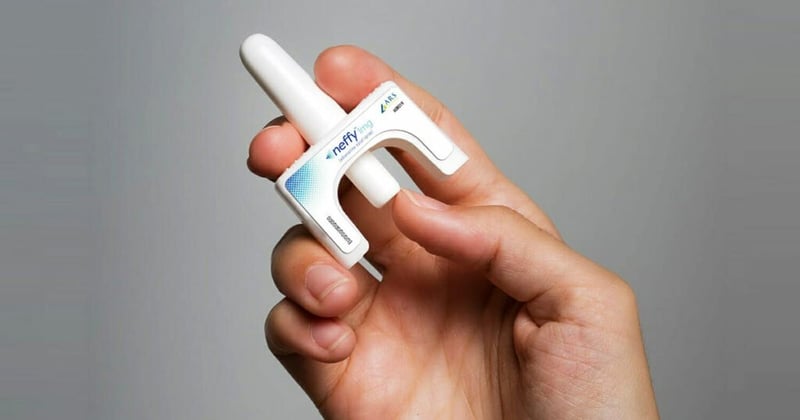
Outside advisors for the U.S. Food and Drug Administration voted Thursday to recommend approval of Neffy, the first epinephrine nasal spray for severe allergic reactions.
Although most of the Pulmonary-Allergy Drugs Advisory Committee members supported the spray for adults (16:6) and children (17:5), key questions linger about whether more data is needed from its maker, ARS Pharmaceuticals, CBS News reported.
But Richard Lowenthal, co-founder, president and CEO at ARS, said in a company statement following the vote that, "We believe our clinical data from more than 600 individuals demonstrate Neffy's absorption-enhancing nasal spray technology is comparable to injectable products in delivering potentially lifesaving epinephrine, but with unique advantages of being small, needle-free and conveniently sized."
Lowenthal added, "We are committed to making it easier for patients and caregivers to carry and administer epinephrine without the anxiety and hesitation associated with using a needle-based device."
Neffy delivers a 2-milligram dose of epinephrine. Instead of large clinical trials, the drug company compared its product to already approved injectable epinephrine products, such as the EpiPen. It showed the results for Neffy were neither substantially higher or lower than injectable epinephrine.
"Patients need options -- with different administration methods -- to facilitate actual epinephrine use in an emergency event,"Dr. Carlos Camargo, a professor of emergency medicine at Harvard Medical School in Boston, said in the company's news release.
"The effects of epinephrine on blood pressure and heart rate are surrogates for efficacy and are important in determining if someone is responding to treatment. With Neffy, blood pressure and heart rate are comparable to EpiPen with a single dose -- and with a second dose of Neffy, increases in systolic blood pressure were statistically higher, even better than revealed in the available data from EpiPen, which is crucial for patients requiring a second dose for a severe allergic reaction,"Camargo added.
Epinephrine has been used since 1901 via injection. It was never put through the same approval process as drugs are now because it was on the market before the FDA existed, CBS News reported.
That means the FDA doesn't have a lot of data it can use to compare Neffy's effectiveness in saving patients who are having a severe allergic reaction.
As a result, it's not entirely clear whether the nasal spray will work as well as injectable epinephrine.
"I would really hate to learn, without some better clinical data, that we recommend approval of a product on the basis of surrogate data that's inconsistent and somewhat confusing and, ultimately because of that, patients are harmed," committee member Dr. Lewis Nelson said, CBS News reported.
But it's also not certain whether more research would really offer additional information.
"What more can we reasonably legitimately expect?" said committee member Dr. Leonard Bacharier. "And if that turned out exactly the way we wanted it, would we have that much greater confidence than where we sit now? I'm just not sure of that."
The FDA is expected to make its decision on whether to approve Neffy by the middle of this year. The company has said it could start selling the spray in late 2023. The drug is designed to work for patients who are about 66 pounds and up.
The company's research included a survey showing that people would be likely to use the spray 18 minutes earlier than an injectable medication, and that as many as 35% more people would carry it with them.
"If they don't have it with them, it's a moot point. They don't deliver, they don't have drug, they go to the hospital," Lowenthal told the committee, CBS News reported.
More information
The American College of Allergy, Asthma and Immunology has more on anaphylaxis.
SOURCES: ARS Pharmaceuticals, news release, May 11, 2023; CBS News
