(330) 876-1228
8507 Main StreetKinsman, OH 44428
(330) 876-1229
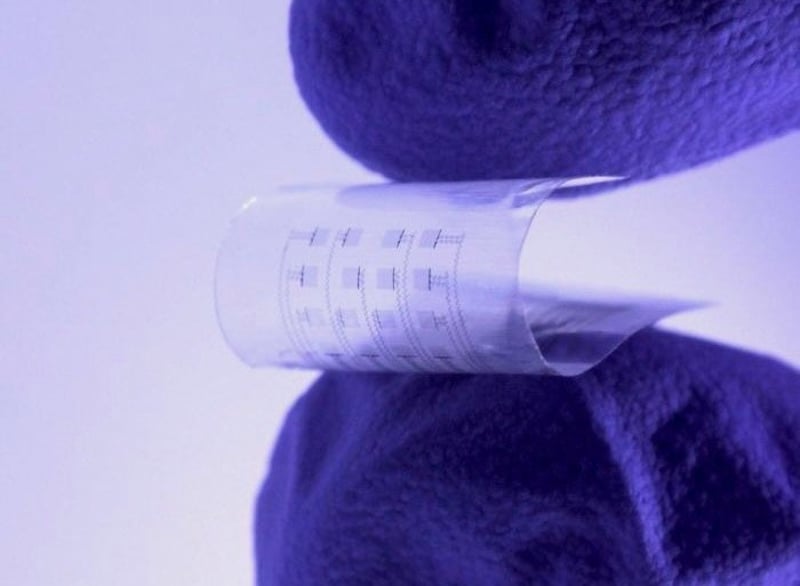
An experimental implant now under development could serve as a temporary monitor and pacemaker for ailing heart patients -- then dissolve away when it's no longer needed.
The soft, lightweight and transparent implant is about the size of a postage stamp, and is made of polymers and metals that are biodegradable, researchers reported July 5 in the journal Science Advances.
Early experiments have shown that the implant can be placed upon the heart of a lab rat, take accurate readings, and then safely dissolve and be absorbed.
The implant would be a boon for patients who have developed heart rhythm complications as a result of a heart attack, surgery or other treatment, said co-senior researcher Igor Efimov, an experimental cardiologist and professor of biomedical engineering at Northwestern University in Chicago.
Those patients now have to wear sticky sensors and tote a bulky monitor so doctors can keep track of their heart as it recovers, Efimov said.
"The challenge with those devices is they're not very comfortable,"he said. "They interfere with daily functioning. For example, you cannot wash in the shower very easily."
The new implant could be inserted during a person's heart surgery or procedure. It would provide data via electrodes and optical sensors, and even could be rigged to deliver an electrical jolt to set straight any irregular heart rhythms that occur, Efimov said.
"Let's say someone just had a heart surgery. After heart surgery, about 30% of patients will get atrial fibrillation [a-fib],"he said. "We want to create an electronic device which can be implanted for the amount of time required, then dissolve."
In cases of postoperative complications like a-fib, devices would typically be required for about 10 days and then no longer be needed, Efimov said.
About one-third of the nearly 700,000 people who die from heart disease each year in the United States succumb to complications in the first weeks or months following a heart attack or heart surgery, researchers noted.
"Many deaths that occur following heart surgery or a heart attack could be prevented if doctors had better tools to monitor and treat patients in the delicate weeks and months after these events take place,"said co-senior researcher Luyao Lu, an assistant professor of biomedical engineering at George Washington University in Washington, D.C.
The device is made entirely of materials that have been deemed safe and biocompatible for humans by the U.S. Food and Drug Administration, Lu said.
For example, the electrodes are made primarily of molybdenum, an element that occurs naturally in the human body, Lu said. The transparent, flexible framework of the device is composed of poly(lactic-co-glycolic acid), or PLGA, a polymer that naturally degrades into harmless chemicals.
"They are all safe and have been approved by the FDA for human use,"Lu said.
Researchers compare the implant to absorbable stitches that degrade and then disappear as the body absorbs them. Patients wouldn't have to undergo follow-up surgeries to either remove or adjust an implant.
Researchers designed the implant to be flexible, so it could be easily placed without causing discomfort, Efimov said.
They also crafted it mainly of transparent materials so that optical sensors in the implant could provide readings on oxygen levels, metabolism and other important measurements, he added.
Currently, many heart patients wind up with a permanent pacemaker when they might not really need one, said Dr. Deepak Bhatt, director of Mount Sinai Heart in New York City.
Others are forced to spend longer periods in a hospital with a temporary pacemaker, then undergo another surgery to have it removed, said Bhatt, who was not involved with the new study.
The experimental implant "could be a really useful solution, either helping a patient to discharge sooner from the hospital instead of having a temporary pacemaker that ties them to the hospital for a period of several days or where they get a permanent pacemaker but then have to deal with that the rest of their life,"Bhatt said.
Bhatt gave the example of someone who undergoes a heart valve replacement.
"Some percentage of those patients, maybe 5 to 10%, will end up needing a pacemaker because their heart rhythm slows down as a consequence of the procedure,"he said. "Potentially with this technology here, which as far as the experiments show is fully bioresorbable, one could envision there being a temporary sort of pacemaker that dissolves in the next several weeks as the heart's native pacemaker function kicks back in."
Instead of being left with a permanent implant, as is now the case, Bhatt said a patient would receive an implant that eventually dissolves.
"To be clear, this is very early research, but the experiments that they did I thought were carefully designed, quite elegant, and showed that these microelectrode arrays could potentially be useful in specific clinical situations," Bhatt said.
The device is still years away from being available for humans, Efimov said.
Efforts are underway to add wireless data transmission to the implant, he said. It then must be tested on larger animals with heart systems akin to those of humans, most likely pigs, before it will be ready for human trials.
"We have a couple of applications which I think realistically we can probably deliver something within three to five years, on relatively simple devices like that,"Efimov said. "More sophisticated devices might take longer."
Once the implant technology is perfected, Efimov can envision it being used to treat many other types of diseases.
For example, temporary implants could track illnesses of the brain, gut and lungs, he said.
"There are so many potential applications, once the technology has been developed,"Efimov said. "You just apply it to different fields of medicine."
More information
The American Heart Association has more about heart implants.
SOURCES: Igor Efimov, PhD, professor, biomedical engineering, Northwestern University, Chicago; Luyao Lu, PhD, assistant professor, biomedical engineering, George Washington University, Washington, D.C.; Deepak Bhatt, MD, MPH, director, Mount Sinai Heart, New York City; Science Advances, July 5, 2023
